Are we going to predict and prevent type 1 diabetes? Yes, we will! Not tomorrow but I expect within a few years, and more research soon!
Last week, the FDA Endocrinologic and Metabolic Drugs Advisory Committee approved the treatment of people at high risk of Type 1 diabetes with Teplizumab for further application and development.
This is a treatment in people (mainly family members for the time being) who are on their way to developing clinical type 1 diabetes, but who not yet show known symptoms or have not yet been treated with insulin. The aim is to inhibit, delay and preferably prevent the clinical onset of type 1 diabetes. Previously the FDA gave Teplizumab the Breakthrough Therapy status and allowed expedited review for this application, in order to further develop this preventive therapy and get it available.
We now know that type 1 diabetes only occurs in people with a (measurable) genetic susceptibility and starts many months/years earlier than the moment when the known clinical signs (frequent drinking/urinating/ weight loss/ etc) become noticeable. The start of the disease process begins, we assume, with a derailment (stress, infection, toxin?) of the insulin-producing beta cells. This creates, among other things, abnormal substances (peptides) against which the well-known ‘auto’-immunity arises, a well-intentioned clean-up action that can, however, severely damage the beta cells (see this review)
In people who go through this disease process, immunological effects become measurable, such as autoantibodies. If this concerns ≥ 2 types of autoantibodies, this is a sign of imminent type 1 diabetes and is indicated by stage 1. This is followed by stage 2 in which abnormal glucoses are also regularly measurable (as sensor measurement, in a milkshake test or a glucose tolerance test) in addition to ≥ 2 autoantibodies. Ultimately, this will result in clinical diabetes (stage 3). It makes sense that intervention as early as possible seems more useful. And that’s what teplizumab can do in stage 2.
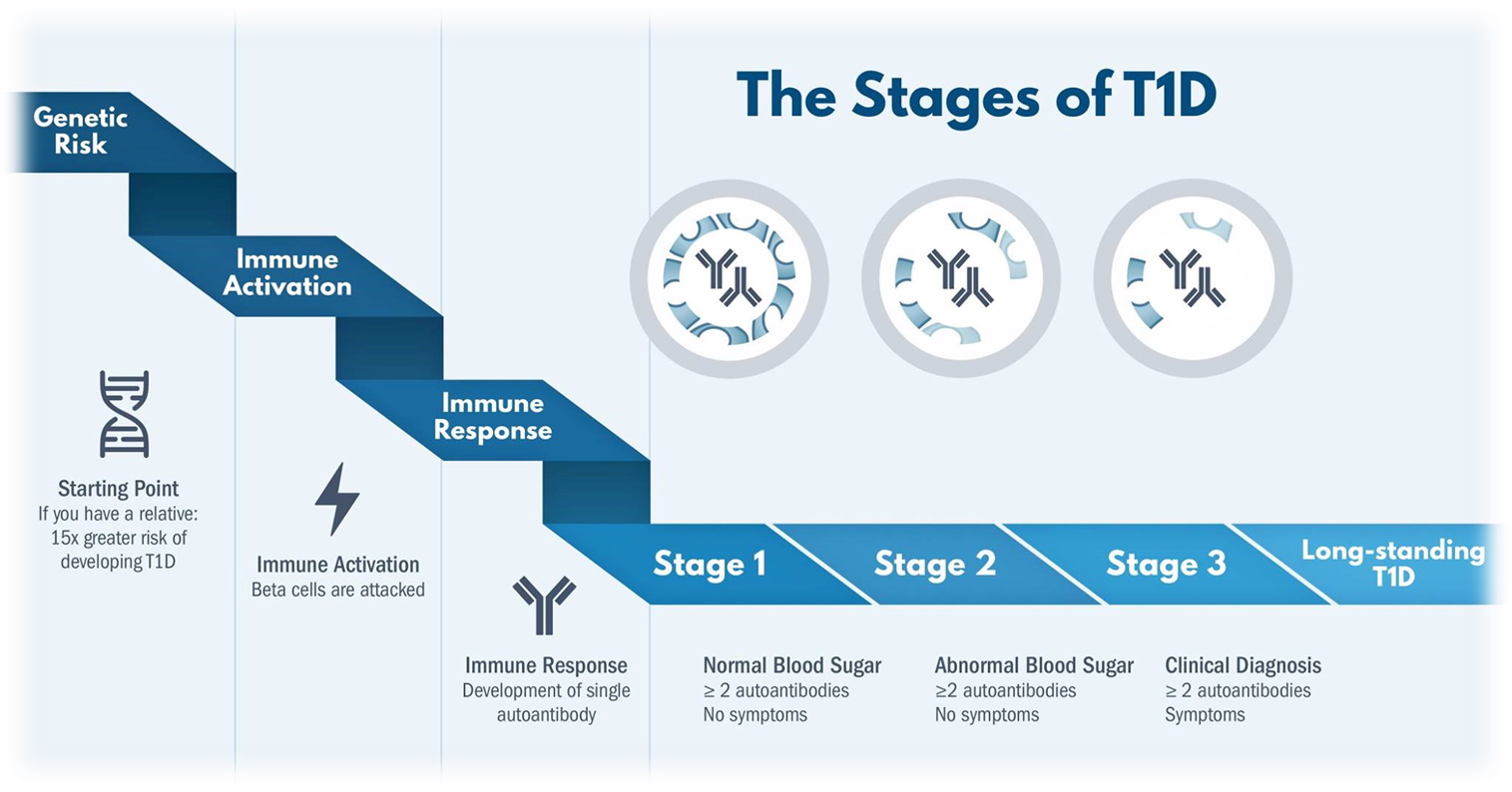
Teplizumab had already been tested with limited success in stage 3. In 2019, the Type 1 diabetes Trial Net Study Group published the results of Teplizumab given at stage 2, i.e. to people on their way to type 1 diabetes (≥ 2 autoantibodies and measurable glucose abnormality). In addition, a one-off (14-day) treatment with Teplizumab slowed clinical signs to approximately 2 years.
It was a small study and there are therefore many questions, but this step by the FDA paves the way for further research and application. For example, there are many practical, medical safety and ethical questions, but the road to prevention of type 1 diabetes is now taken seriously!